Process and Analytical Method Development and Validation
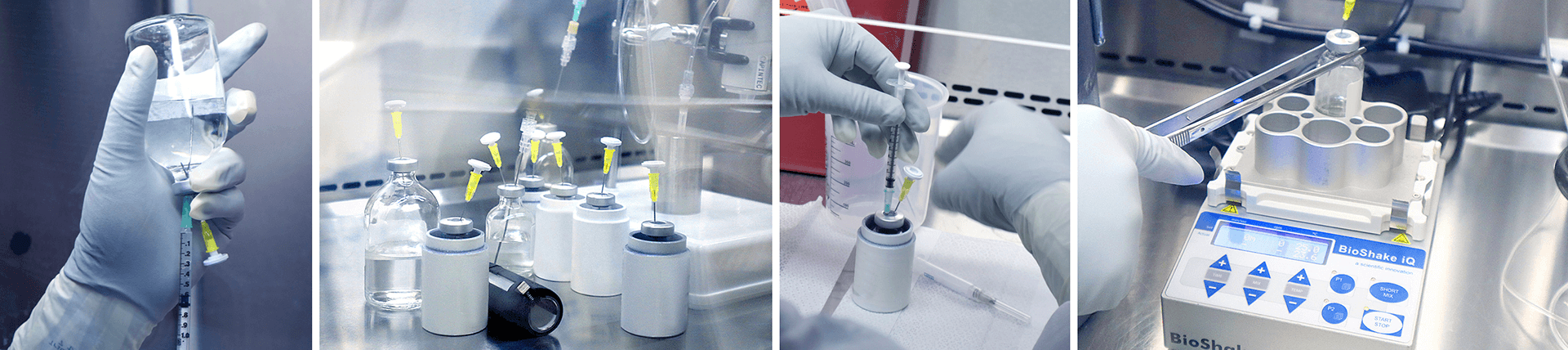
AtomVie has in-depth experience in GMP radiopharmaceutical manufacturing, including expertise in radiolabeling with an array of radioisotopes such as Lu-177, Ac-225, I-131, In-111, and Zr-89 and ligands that range from small molecules, peptides, to small and large antibodies.
To support our clients we provide a range of services from early process development, tech transfer, process and formulation optimization, to analytical methods, scale-up and batch development. Our sophisticated subject matter experts in Process and Analytical Method Development and Validation have more than a decade of expertise in scale-up and stability studies.



Quality
Operations
Whether we are conducting a tech-transfer or designing a process from scratch, AtomVie builds quality into your program from its inception.
Our seasoned Quality Control, Quality Assurance, and Microbiology teams work closely with our clients to understand their unique needs and ensure that all of our programs are conducted in accordance with current Good Manufacturing Practices (cGMPs) guidelines. Facilities operate in accordance with commercial Canadian, US, and EU GMP requirements.
5+
Audits were done by regulatory agencies and clients in 2022
cGMP
Compliant Quality System
45+
We have more than 45 quality professionals working in our Quality Operations Department
Regulatory
AtomVie’s Regulatory Affairs Department has collectively >20 years of experience successfully registering investigational and commercial radiopharmaceuticals.
AtomVie's Regulatory Team has a success rate of 100% filing over 300 submissions to Health Canada and the FDA. Our team has experience working with both diagnostic and therapeutic products as well as small and big pharma clients. The Regulatory Team leverages its close relationship to other AtomVie departments to ensure efficient preparation of submissions. We offer a variety of regulatory services including:
- Preparing and reviewing CMC sections
- Preparing, publishing, and acting as your regulatory agent for filings
- Preparing and reviewing clinical trial documents (Investigator's brochure, clinical trial protocols, Patient Information and Consent Forms (ICFs), and product monographs)
- Providing regulatory advice on product labeling and design
- Performing gap analyses of regulatory strategies and submissions
- Assessing, strategizing and submitting post-clearance changes
Diverse Distribution of Clinical Trial Phases of Sponsored Programs at AtomVie

Clinical Supply
We have shipped over 1020 doses to over 15 countries globally in 2022
Our team has a successful track record of clinical supply for phases I to III clinical trials across the globe for more than a decade.
We understand the importance of manufacturing reliability in the radiopharmaceutical industry, which is why AtomVie departments work together to secure reliable clinical supply for all of our clients.
Our team has achieved a 99.5% success rate in shipping radiopharmaceuticals worldwide
Commercial Supply
AtomVie's new facility will be a state-of-the-art, multi-product facility that will be comissioned in 2024. The new facility will:
- Have the potential for 10+ times more capacity than the current facility.
- Be compliant with commercial Canadian, US and EU GMP requirements.
- Have capacity to work with I-131, Lu-177, Ac-225, Tb-161, In-111, Y-90 plus other isotopes on a client need basis.

Isotope Supply
Non-carrier added (n.c.a.) Lu-177 supply to North America in collaboration with Isotopia Molecular Imaging.

Logistics
We deliver to over 15 countries on a daily basis across North America, South America, Europe, Asia, Africa, and Australia!
We will design and implement an effective and comprehensive strategy to optimize the delivery of your radiopharmaceutical product to its final destination. AtomVie's logistics team will find the best and most reliable mode of transportation based on your needs to guarantee just in time treatments of patients.







